
If Science Can Cure Sickle Cell Disease, Are We Ready to Pay For It?
1. Understanding Sickle Cell Patients’ Immense Suffering Today
Nearly 100,000 individuals in the United States have Sickle Cell Disease (SCD), an inherited disorder caused by a mutation in the globin gene responsible for making red blood cells. Roughly 70% of people with SCD are on Medicaid, 85%+ are black1, 40% are children and adolescents, and many have inadequate access to healthcare.
SCD has historically received insufficient attention from policymakers and the medical community, leading to a shortage of drugs and facilities: SCD research obtained significantly less research funding per person compared to diseases like cystic fibrosis (Farooq et al., 2020)2, and there are not nearly enough hematologists specialized in Sickle Cell to meet patient needs3. The only available SCD cure is a bone marrow transplant from a matched donor (most often a fully matched sibling), and is typically administered to younger children who are more tolerant of such procedures.
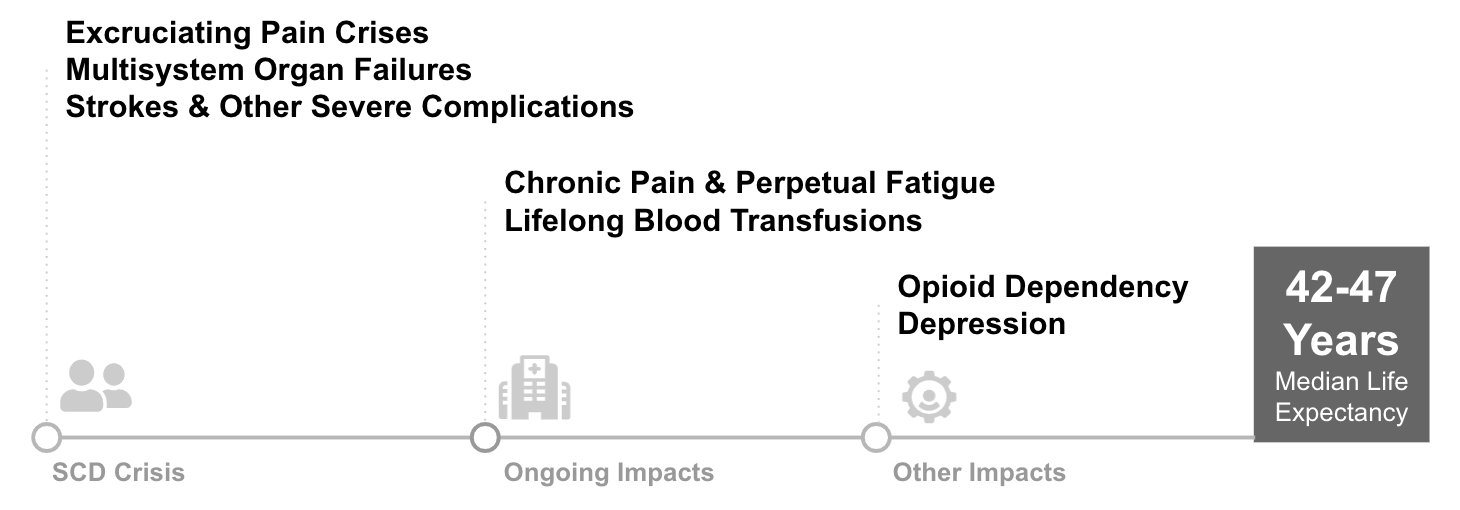
In the absence of a highly effective therapy, patients suffer from debilitating symptoms and significant risk of early death. As the disease progresses, patients may face frequent extreme pain episodes requiring hospitalization, organ damage, and strokes that can be fatal. These extreme symptoms often have secondary impacts on patients’ lives: opioid addiction related to disease pain is fairly common (10%)4, unemployment and disability rates are high compared to siblings without SCD (Idowa et. al, 2013)5, depression rates are 33%, or roughly 350% of the population average6. Median lifespan is roughly 42-47 years old while the U.S. population median is 79. Surveys of patients highlight the tremendous suffering and need for better therapies: multiple papers have found over 60% of patients would tolerate a ~15% procedure mortality risk in order to be cured.
2. A New Hope - Gene Therapy
Since 2019, several trials of a gene therapy treatment for sickle cell disease have demonstrated astonishing results among a select group of participants, raising the hope that a transformative treatment could soon be at hand. In the recent bluebird bio phase 3 trial, 35 patients between the ages of 12 to 40 were dosed with the gene therapy. Nearly all of them saw a 100% reduction in vaso-occlusive events (VOE) as well as sustained levels of healthy HbA.7
Gene therapy holds promise for the approx 20% of all SCD patients who suffer from severe SCD, encountering 3+ several VOE pain crises per year that require hospitalization and blood transfusions and significantly increases the risk of stroke and complications.
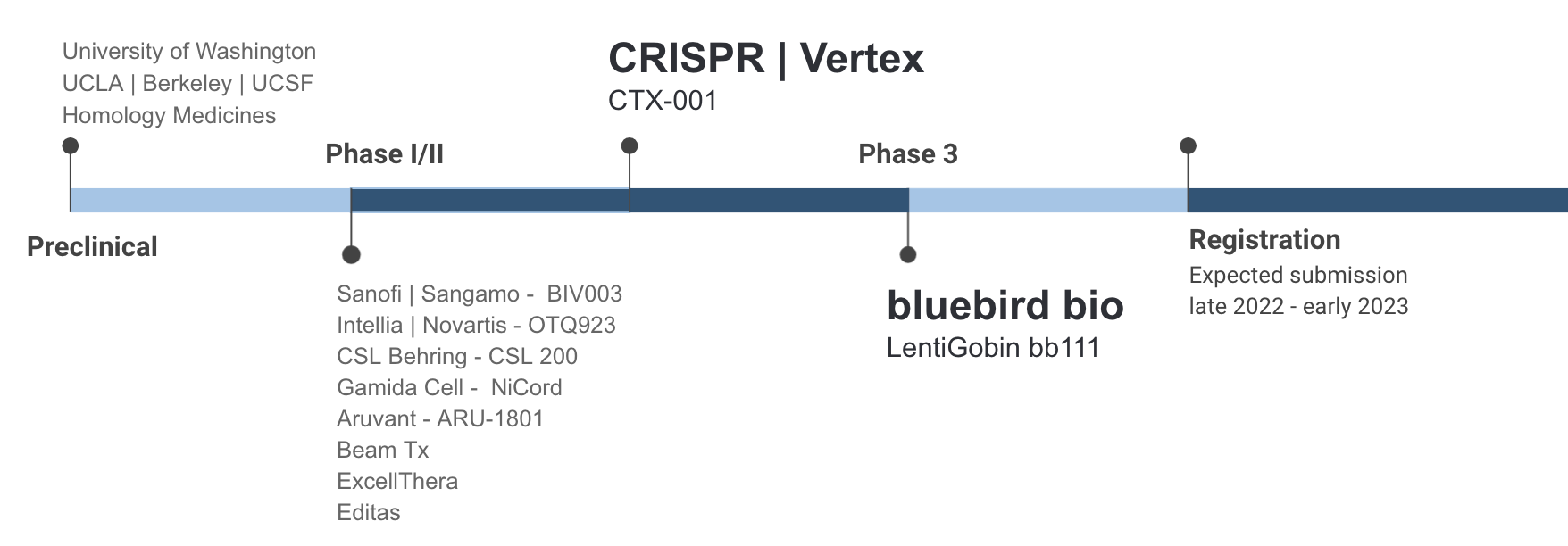
As encouraging as the pipeline may seem, the potential of the science is clouded by uncertainty over whether SCD patients can even access these treatments if they are approved, and at what cost. Prices for gene therapy currently range between $800K (Luxturna, two eyes) and $2.1M (Zolgensma, per patient). At these price points, patients and payers alike could find affording these treatments an insurmountable burden.
Gene therapy is not perfect and will not be suitable for every patient. The treatment requires a round of myeloablative chemotherapy to prevent blood cell formation, which commonly causes infertility and also precludes patients with end-organ damage. Patients who have had a stroke also do not qualify for current gene therapy trials. There is also the risk of developing myeloid malignancies caused by either the busulfan treatment or lentiviral vector. In addition, the long-term durability of gene therapy has yet to be proven, with the longest follow-ups in clinical trials around 24 months.
3. Will Gene Therapy Be Worth Its Price?
Unlike traditional drugs, gene therapy offers the potential opportunity for a one-time highly effective treatment, but at an extremely steep price. The big question is - will the therapy be cost effective for payers, especially for Medicaid who insures nearly 70% of all SCD patients? To answer this, we examined the cost of care for patients living with severe SCD, who are expected to be the primary target population for a gene therapy treatment.
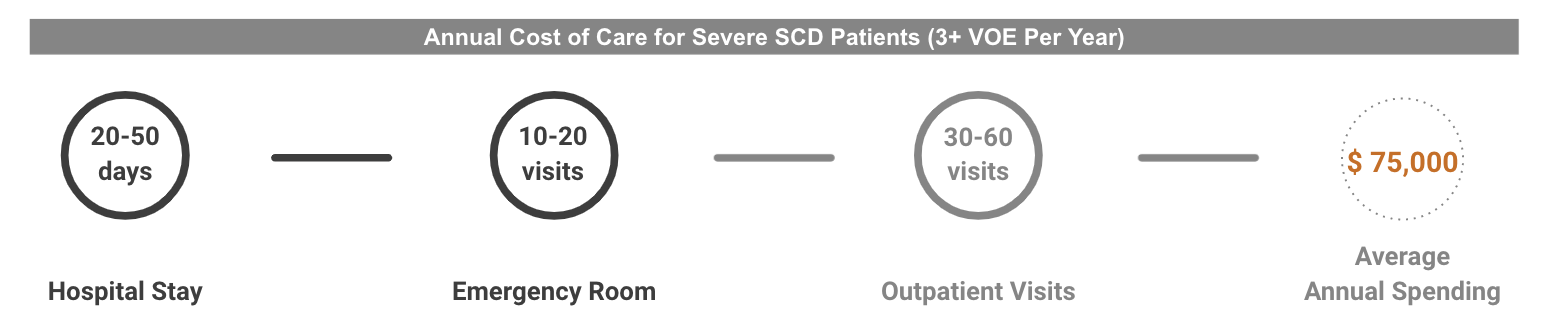
The annual medical cost for SCD patients with multiple pain crises (3+ VOE/year) is roughly $75,000 (Shah et. al 2020 adjusted by inflation)8. Over the course of 40 years, the medical spending alone amounts to nearly $3M per patient. And in addition to the cost of treating symptoms and associated complications, patients with sickle cell anemia also face severe economic challenges, with estimates of income loss around $15K per individual per year (Holdford et al. 2021). There is also a significant financial burden on families, with caregivers also reporting significant income loss associated with the disease. If SCD patients could be treated at early ages, even the benefit of such a drastic improvement in life quality alone could largely exceed the cost of gene therapies at their current price points.
4. Will Medicaid be able to Provide Sufficient Access to Transformative Treatments for SCD Patients?
Medicaid insures over 70% of SCD patients in the US. Each state administered health plan plays a key role in patient access for SCD GCT products. In order to gain an comprehensive understanding of challenges Medicaid may face in the near future when it comes to budgeting and treatment logistics, we examined the following 3 questions:
- How will gene therapies be adopted by current SCD patients once approved ?
- What is the budgeting impact on each state Medicaid plan?
- Are there sufficient facilities to ensure adequate access for patients ?
4.1 On Adoption: Will Patients Choose Treatment, and How Quickly?
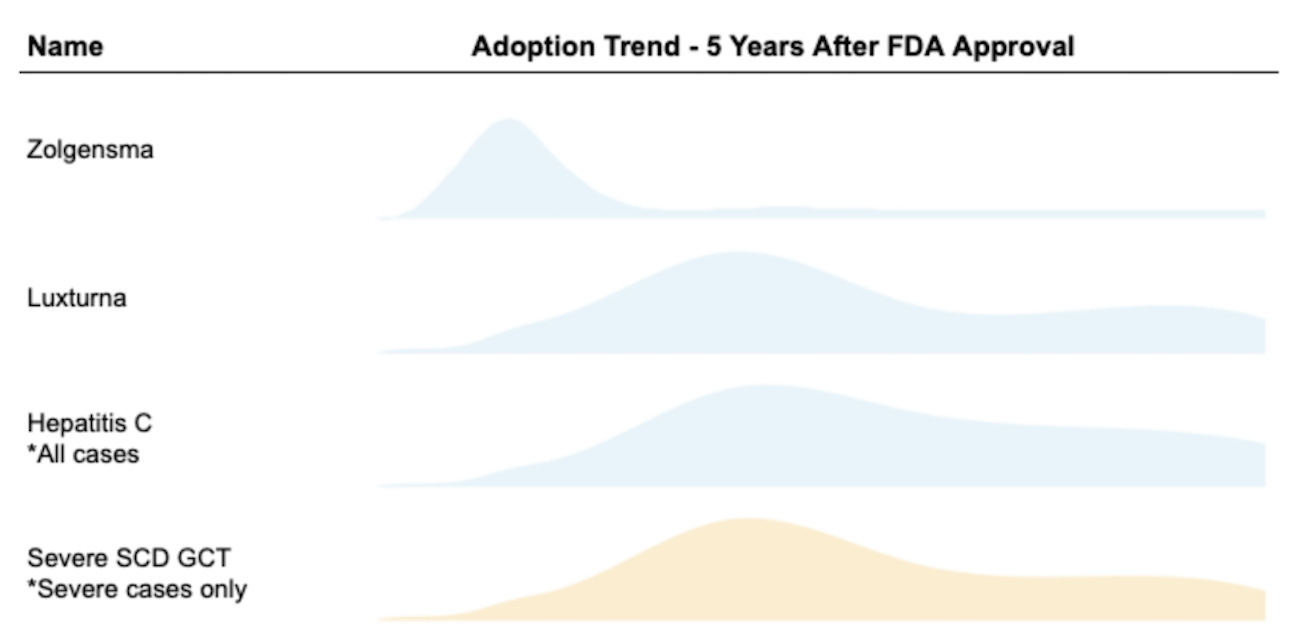
Patients with sickle cell anemia have often expressed a strong willingness to take mortality risks for a potential cure. Past surveys like Van Besien et. al (2001)9 and Chakrabarti and Bareford (2007)10 found that over 60% of SCD patients would accept some mortality risk in order to receive a bone marrow transplant, and 15% of them were willing to accept a risk of death as high as 35%. In comparison to the stem cell transplant option, gene therapies are potentially safer and possibly more efficacious: for these reasons, we suspect that the proportion of eligible patients who get gene therapy will be higher than the proportion of eligible patients who get stem cell treatment. Expert opinion - Goldman and Leising (2021)11, Teixeira et. al (2019)12, and Quinn et. al (2019)13 - also generally supports the notion that a transformative therapy will attract a large share of eligible patients. This mix of expert opinion and survey data leads us to make a base case estimate of 70% adoption over five years.
For Medicaid finances, how that 70% is distributed across years will matter a great deal. In order to estimate the demand curve for SCD GCT product with limited data available, we also took the following products’ historical adoption trends into consideration:
- Novartis’ SMA gene therapy Zolgensma approved in 2019
- Roche/Spark’s LCA gene therapy Luxturna approved in 2017
- Other curative drugs such as Hepatitis C treatment adoption (TC Feldman 2021)14
We estimate the majority of eligible patients with severe SCD will opt-in for the gene therapy within 5 years, with demand peaking in year two after FDA approval based on our qualitative analysis.
4.2 On Medicaid Budgeting: Can States Afford It?
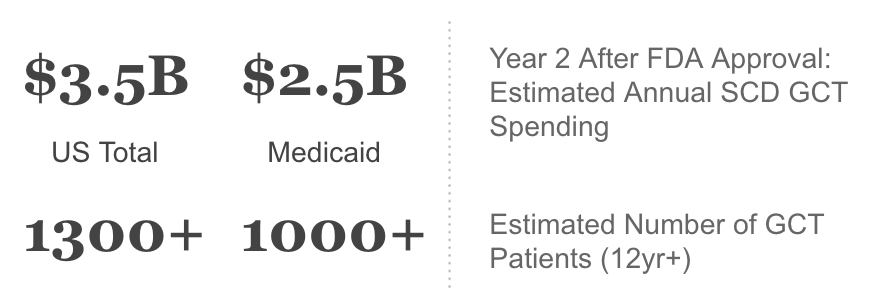
The introduction of transformative GCT therapies for SCD is likely to put significant strain on State Medicaid plans. The table below shows our base case for Per-Member-Per-Month (PMPM) costs across states in the second year after FDA approval under two scenarios:
- FDA approval for ages 12+ (12-50 Trial15)
- FDA approval for ages 3+ (Aged 3-50 trial16, 3-40 trial17)
States like Mississippi and South Carolina could see costs of as high as $4.00 PMPM - $11.00 PMPM for the therapy; whereas states with lower prevalence like Utah & West Virginia will face much smaller hits. These projections assume that states will not erect significant barriers to care for a transformative therapy.
Budget impact in the range of $6-$11 PMPM is nearly unprecedented for a single therapy. In comparison, the gene therapy Zolgensma accounts for $0.20-0.30 pmpm for Medicaid. The wave of Hepatitis C cures in 2014-2016 led to large budgeting constraints among states’ Medicaid plans, with costs of on average $1.50 pmpm across states with the highest HepC prevalence18. A cost increase of $5 PMPM represents a greater than 1% increase in overall spend for most states. Whether states can afford this increase will likely depend on the Federal Government’s willingness to help most impacted states, and whether States and pharmaceuticals can come up with solutions to spread the up-front costs over time. If this does not occur, States may erect barriers to care in the face of very high costs. Patients and their families would ultimately bear the brunt of such failure.
4.3 On Network Adequacy: Will Hospitals Have Enough Capacity?
Gene and Cell Therapy for Sickle Cell requires sophisticated infrastructure and expertise that only a handful of facilities are capable of providing. On clinicaltrials.gov, thirty-one facilities are listed as being involved in a current trial for GCT for Sickle Cell, and these facilities are concentrated in California and the Northeast, far from Southeastern states with the highest patient prevalence. While the number of facilities that can administer GCT is likely greater than those involved in the trials, physician experts surveyed believe the list of facilities will be initially pretty limited.
States with the highest SCD patient prevalence such as Alabama, Mississippi, and Louisiana likely have zero or one in-state facilities. In order to meet the patient demand, Medicaid will inevitably need to contract out-of-state centers of excellence. Pharmaceuticals may choose to offer reimbursement for travel costs. However, whether State Medicaid plans are willing to pay for out-of-state services, and whether centers of excellence are willing to accept out-of-state Medicaid patients, will both play key roles in patient access. Private network of facilities and improved public investment in infrastructure will be needed.
5. Conclusion: How do we collectively ensure patient access?
If early clinical trial results hold up, there will soon be a Sickle Cell therapy that is considerably more widely available and safer than the best existing treatment. However, access to this treatment is shadowed by both financial and logistical challenges faced by the healthcare system. How will a dozen budget-constrained state Medicaid programs be able to pay for a sharp uptick in demand for a very expensive therapy? How will healthcare providers be able to meet the need given the facility constraints? Ultimately, will the federal government, the states, and CMS work collaboratively to get ahead of these looming challenges? The next two to three years represents a large potential opportunity for a variety of public and private entities to transform the lives of tens of thousands of people. Coordination and creativity will be crucial to realizing that promise.
If you have any questions about this analysis, or want to learn more about Quantile, feel free to contact us.
Appendix
Methodology
Section 4.2 of this analysis is replicable with publicly available data, and we encourage readers to reproduce the results with our methodology listed below.
We applied Sickle Cell in Medicaid prevalence studies18 to current Medicaid enrollment numbers to obtain the model Medicaid SCD patient population20. Next we added in an estimate of 20% of sickle cell cases being severe, and used our modeled adoption curve to estimate % uptake in Year 2 after product launch. The analysis also assumed that a gene therapy treatment for SCD would be priced similarly to Zolgensma ($2M) or bluebird bio’s Zyngelto for beta-thal ($1.8M).
The range of Medicaid pmpm costs were estimated using the number of patients expected given approvals for different age ranges. The age ranges were obtained from clinical trial information, and combined with the distribution of Medicaid Sickle Cell patients by age19. This resulted in the Medicaid budget estimates displayed in Table 3.
Other authors18 have also estimated the SCD GCT financial impact on state Medicaid plans, and concluded a lower PMPM based on a significantly more conservative set of assumptions on patient adoption.
References
[1] MediLexicon International. (n.d.). Sickle cell anemia in African Americans: Symptoms, causes, and more. Medical News Today. Retrieved November 22, 2021, from https://www.medicalnewstoday.com/articles/african-american-anemia#causes.[2] Farooq F, Mogayzel PJ, Lanzkron S, Haywood C, Strouse JJ. Comparison of US Federal and Foundation Funding of Research for Sickle Cell Disease and Cystic Fibrosis and Factors Associated With Research Productivity. JAMA Netw Open. 2020;3(3):e201737. doi:10.1001/jamanetworkopen.2020.1737
[3] Marcus, E. (2016, March 21). Our healthcare system abandons adult sickle cell patients. The Washington Post. Retrieved November 22, 2021, from https://www.washingtonpost.com/national/health-science/unbearable-pain-not-enough-doctors-just-another-day-as-an-adult-with-sickle-cell/2016/03/21/f6598a5c-e655-11e5-a6f3-21ccdbc5f74e_story.html.
[4] Nhlbi.nih.gov. 2021. Opioid crisis adds to pain of sickle cell patients | NHLBI, NIH. [online] Available at: https://www.nhlbi.nih.gov/news/2017/opioid-crisis-adds-pain-sickle-cell-patients [Accessed 22 November 2021].
[5] Modupe Idowu, Omotayo Fawibe, Paul Rowan, Harinder S. Juneja, Deborah Brown; Occupational History For Adults With Sickle Cell Disease Compared With Healthy Siblings. Blood 2013; 122 (21): 2244. doi: https://doi.org/10.1182/blood.V122.21.2244.2244
[6] Jenerette, C. M., & Brewer, C. (2010). Health-related stigma in young adults with sickle cell disease. Journal of the National Medical Association, 102(11), 1050–1055. https://doi.org/10.1016/s0027-9684(15)30732-x
[7] “New Data Show near Elimination of Sickle Cell Disease-Related Vaso-Occlusive Crises and Acute Chest Syndrome in Phase 1/2 Clinical Study of Bluebird Bio's Lentiglobin™ Gene Therapy for Sickle Cell Disease at 25th EHA Congress.” Bluebird Bio, Inc., https://investor.bluebirdbio.com/news-releases/news-release-details/new-data-show-near-elimination-sickle-cell-disease-related-vaso.
[8] Shah N, Bhor M, Xie L, Paulose J, Yuce H. Medical Resource Use and Costs of Treating Sickle Cell-related Vaso-occlusive Crisis Episodes: A Retrospective Claims Study. JHEOR. 2020;7(1):52-60. doi:10.36469/jheor.2020.12852.
[9] van Besien K, Koshy M, Anderson-Shaw L, Talishy N, Dorn L, Devine S, Yassine M, Kodish E. Allogeneic stem cell transplantation for sickle cell disease. A study of patients' decisions. Bone Marrow Transplant. 2001 Sep;28(6):545-9. doi: 10.1038/sj.bmt.1703208. PMID: 11607766.
[10] Chakrabarti S, Bareford D. A survey on patient perception of reduced-intensity transplantation in adults with sickle cell disease. Bone Marrow Transplant. 2007 Apr;39(8):447-51. doi: 10.1038/sj.bmt.1705622. Epub 2007 Mar 5. PMID: 17334383.
[11] Leising, Jordan Michael, and Olivia Claire Goldman. "Portfolio Modeling and Forecasting of Single-Use Rare Disease Treatments." (2021).
[12] BCG Global. 2021. From Lab to Marketplace, Succeeding With Gene Therapies. [online] Available at: https://www.bcg.com/publications/2019/lab-marketplace-succeeding-gene-therapies [Accessed 22 November 2021].
[13] Quinn C, Young C, Thomas J, Trusheim M; MIT NEWDIGS FoCUS Writing Group. Estimating the Clinical Pipeline of Cell and Gene Therapies and Their Potential Economic Impact on the US Healthcare System. Value Health. 2019 Jun;22(6):621-626. doi: 10.1016/j.jval.2019.03.014. Epub 2019 May 17. PMID: 31198178.
[14] Feldman, Theodore & Dienstag, Jules & Mandl, Kenneth & Tseng, Yi-Ju. (2021). Machine-Learning-Based Predictions of Direct Acting Antiviral Therapy Duration for Patients with Hepatitis C. International Journal of Medical Informatics. 154. 104562. 10.1016/j.ijmedinf.2021.104562.
[15] “A Study Evaluating the Safety and Efficacy of BB1111 in Severe Sickle Cell Disease - Full Text View.” Full Text View - ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02140554.
[16] Clinicaltrials.gov. 2021. A Study Evaluating Gene Therapy With BB305 Lentiviral Vector in Sickle Cell Disease - Full Text View - ClinicalTrials.gov. [online] Available at: https://clinicaltrials.gov/ct2/show/NCT04293185 [Accessed 22 November 2021].
[17] Clinicaltrials.gov. 2021. Gene Transfer for Sickle Cell Disease - Full Text View - ClinicalTrials.gov. [online] Available at: https://clinicaltrials.gov/ct2/show/study/NCT03282656 [Accessed 22 November 2021].
[18] DeMartino P, Haag MB, Hersh AR, Caughey AB, Roth JA. A Budget Impact Analysis of Gene Therapy for Sickle Cell Disease: The Medicaid Perspective. JAMA Pediatr. 2021 Jun 1;175(6):617-623. doi: 10.1001/jamapediatrics.2020.7140. Erratum in: JAMA Pediatr. 2021 Jun 1;175(6):647. PMID: 33749717; PMCID: PMC7985816.
[19] Wilson-Frederick S, Hulihan M, Kai Anderson K. Prevalence of Sickle Cell Disease among Medicaid Beneficiaries in 2012. Centers for Medicare and Medicaid Services, Office of Minority Health. 2016. https://www.cms.gov/About-CMS/Agency-Information/OMH/Downloads/Data-Highlight-16-Sickle-Cell-Disease.pdf.
[20] Medicaid & CHIP Enrollment. (2021, November 9). KFF. https://www.kff.org/state-category/medicaid-chip/current-medicaid-chip-enrollment/.