Patient Advocacy Spotlight: Little Hercules Foundation Tackles Gene Therapy Denials
Nov 4, 2024
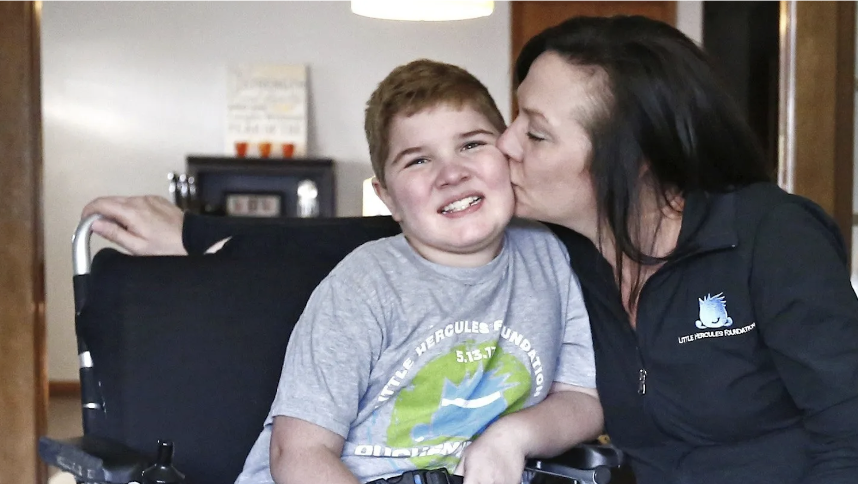
Patient advocacy organizations play a pivotal role in ensuring patients have access to life-saving treatments. Kelly Maynard founded the Little Hercules Foundation ten years ago after her son was diagnosed with DMD. Since the approval of Elevidys1, a $3.2 million gene therapy, the foundation has focused on helping families overcome insurance denials and secure access to the life-saving treatment.
Through the foundation’s work, Maynard has identified a crucial issue: 70% of all Elevidys denials come from self-insured employers2 —companies that cover their employees' medical expenses directly instead of using traditional insurance. This is largely due to their inability to afford the high costs associated with these treatments. While the LHF team has successfully overturned 100% of these denials, the appeals process can be painstakingly long, often taking several weeks to months.
The fee-for-service model, which is simply not working for gene therapies, is a market inefficiency with devastating consequences. In human terms, this means children with fatal conditions and patients with excruciating pain are denied life-saving treatments. The approval of Casgevy and Lyfgenia for sickle cell disease this past December makes the need for a new payment model even more pressing.
Maynard notes, “the current process of fighting denials delays access to care and places a heavy burden on patients and their families. It is an approach that is reactive when we need to ensure patients have coverage from the very beginning.” For children with DMD—of which there are approximately 15,000 patients3 in the U.S.—treatment delays can lead to irreversible muscle degeneration and fatal complications.
As more gene therapies for previously fatal conditions receive approval, it is crucial to prioritize patient access in this self-insured employer segment of the US healthcare market and ensure that life-saving treatments are accessible to all patients and families.
To support the Little Hercules Foundation's mission or learn more about their impact, visit their website.
To learn more about Quantile’s subscription based access solution that solves for these challenges, reach out to us at our Contact Page
1 https://www.fda.gov/vaccines-blood-biologics/tissue-tissue-products/elevidys
2 https://www.bloomberg.com/news/articles/2024-09-05/high-gene-therapy-costs-have-employers-refusing-workers-coverage
3 https://cureduchenne.org/about/what-is-duchenne